The IOTN currently consists of the following components:
Cancer Immunotherapy Consortium (CIC): The CIC will be composed of organ site-specific Cancer Immunotherapy Research Projects (supported by RFA-CA-17-045, U01) and Cancer Immunoprevention Research Projects (supported by RFA-CA-17-046, U01). The CIC will form an integrated network of multi-disciplinary, collaborative teams with the overarching goals of accelerating the development of improved immunotherapeutic strategies capable of eliminating established cancers or preventing cancers before they occur. A total of 12 U01s, including 10 Immunotherapy Projects and 2 Immunoprevention Projects are awarded in FY18. The CIC was expanded in FY19 with the addition of 6 Immunotherapy Projects (supported by RFA-CA-19-015, U01) and 3 Immunoprevention Projects (supported by RFA-CA-19-014, U01 and RFA-CA-19-012, UG3/UH3, respectively).
Data Management and Resource-Sharing Center (DMRC): The DMRC (supported under RFA-CA-17-047, U24) will provide overall support, and facilitate collaboration among the adult IOTN-funded components. The DMRC will also promote scientific outreach with other Cancer Moonshot initiatives and the larger scientific community. One DMRC U24 is awarded in FY18.
Cellular Immunotherapy Data Resource (CIDR): The CIDR (supported under RFA-CA-17-048, U24) will support a data registry for collecting outcomes of patients receiving cellular immunotherapies, that could be utilized for observational studies or inform subsequent pre-clinical studies and clinical trials. One CIDR U24 is awarded in FY18.
Immuno-engineering to Improve Immunotherapy (i3) Centers: As new components of the IOTN, the i3 Centers (supported by RFA-CA-19-013, U54) will be comprised of multi-disciplinary teams focused on developing and employing engineered immunotherapy approaches to design more durable, widely accessible, and less toxic immunoprevention and immunotherapy strategies. Four U54 i3 Centers are awarded in FY19.
Advancing Cancer Immunotherapy by Mitigating Immune-Related Adverse Events (irAE): As new components of the IOTN, the irAE Research Projects (supported by RFA-CA-19-044, U01) are led by single investigators and/or multidisciplinary teams with expertise in mechanisms of cancer immunology, immune tolerance, irAEs, autoimmunity, and/or patient characterization and selection. The specific objectives are to generate new ideas and approaches to better understand and thereby reduce the incidence and/or severity of irAEs resulting from cancer immunotherapy. Four U01 irAE projects are awarded in FY20.
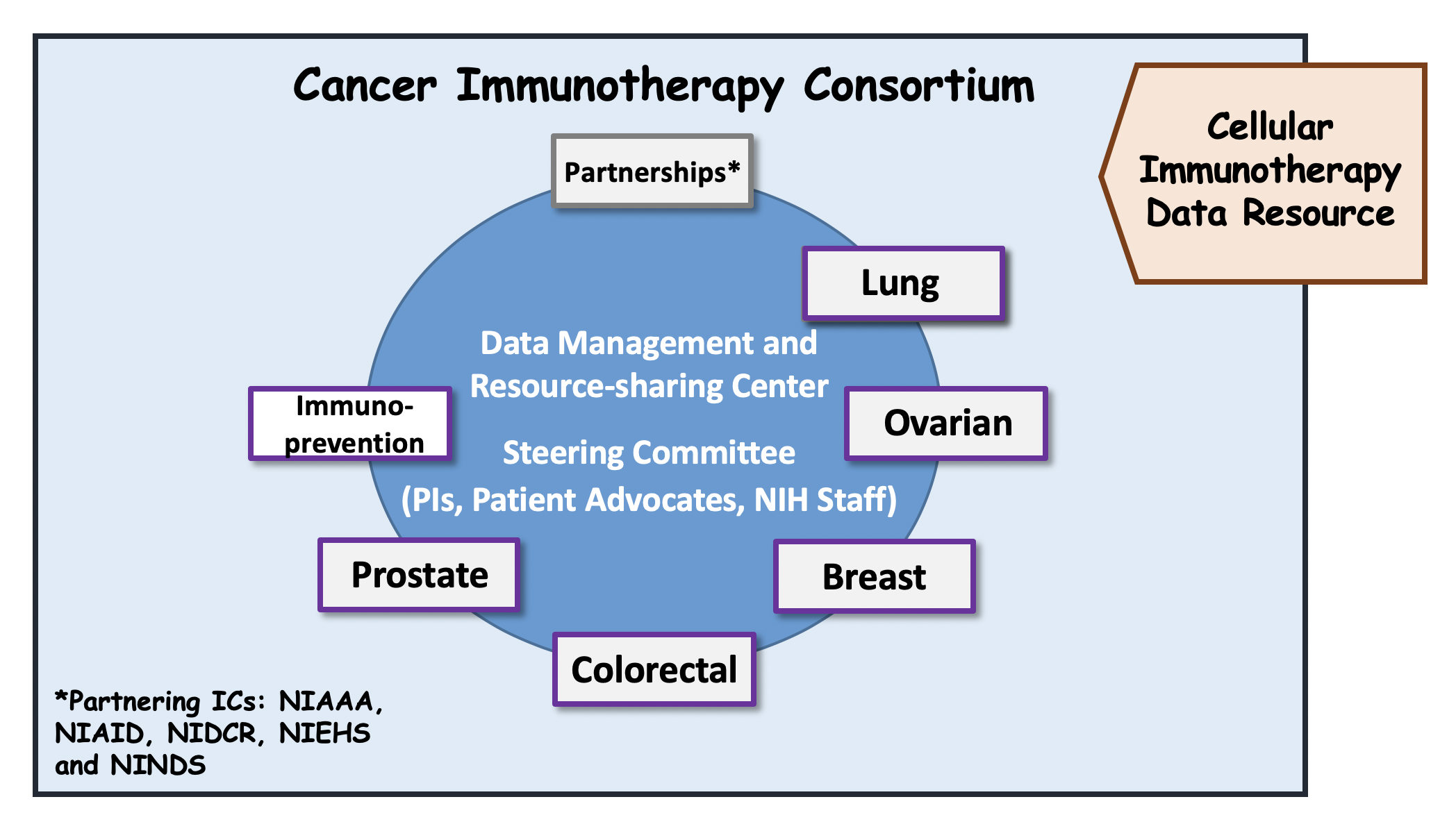
Governance of the IOTN: The NIH program officers for IOTN include Drs. Mansoor Ahmed, Ricardo Cibotti, William Dunty, Lori Henderson, Kevin Howcroft, Chao Jiang, Lillian Kuo, Mark Miller, Gary Murray, Elad Sharon, Terez Shea-Donohue, Connie Sommers, Minkyung Song, Aynur Unalp-Arida, Chiayeng Wang. The IOTN, including the DMRC, CIDR, i3 Centers, irAE and Cancer Immunotherapy and Immunoprevention Research Project components will be governed by the IOTN Steering Committee. The IOTN Steering Committee consists of PIs of U01/UG3/U54/U24 projects, NIH Project Scientists, and representative of a patient advocacy group. The Chair and co-Chair of the IOTN Steering Committee are Drs. Alan Hutson and Kunle Odunsi.